- Full Atomistic MD
- Molecular structure / Affinity / Solubility
- Materials Science
- Life Science
[Analysis Example] Solubility for water
Evaluation of solvation free energy by Free Energy Perturbation method
Solubility is a quantity that quantifies the degree to which a solute dissolves in a solvent. Solubility is measured on a macroscale. Solvation free energy is evaluated when considering solubility in the microscale. The solvation free energy is the difference in free energy between the solute in a vacuum and in a solution, which can be evaluated by Free Energy Perturbation (FEP).
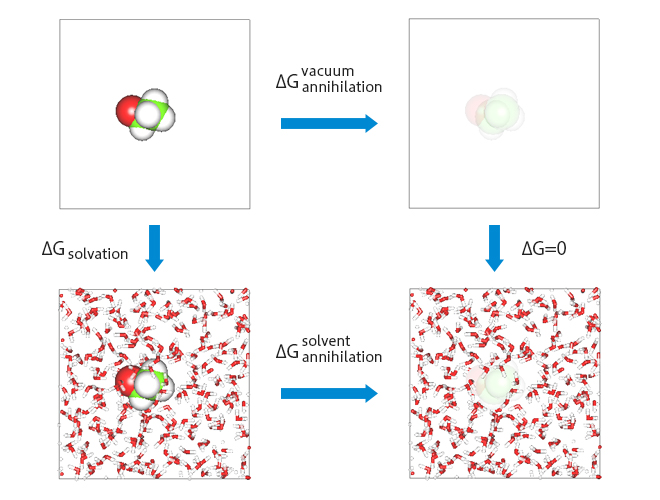 Fig.1:Scheme for evaluation of hydration free energy
Fig.1:Scheme for evaluation of hydration free energy
It would be easy if the solvation free energy could be evaluated by the energy difference between just the two states of that in the vacuum and in the solution. Due to the error in sampling the energy, in practice, intermediate states are inserted between the two states to "eliminate" the solute molecules to achieve more moderate thermodynamic processes.
As shown in Figure 1, the free energy difference between the free energy ![]() in the process of eliminating the solute in the vacuum and the free energy
in the process of eliminating the solute in the vacuum and the free energy ![]() in the process of eliminating the solute from the solution,
in the process of eliminating the solute from the solution, ![]() =
= ![]() −
− ![]() , is evaluated as the solvation free energy
, is evaluated as the solvation free energy ![]() .
.
The molecular dynamics engine GENESIS[1][2] has a function to perform FEP. In this case study, GENESIS was conducted to evaluate the solvation free energy ![]() for five solutes (methanol, ethanol, 2-nitroaniline, aspirin, and caffeine). GAFF was assigned as the force field for the solutes and the OPC3 model was applied for water molecules.Figure 2 shows a graph comparing the results calculated using GENESIS with experimental values[3].
for five solutes (methanol, ethanol, 2-nitroaniline, aspirin, and caffeine). GAFF was assigned as the force field for the solutes and the OPC3 model was applied for water molecules.Figure 2 shows a graph comparing the results calculated using GENESIS with experimental values[3].
The calculated results are in good agreement with experimental values. The function for evaluating solvation free energy by FEP is implemented in J-OCTA/GENESIS modeler, which is an important property in drug discovery and cosmetic design.
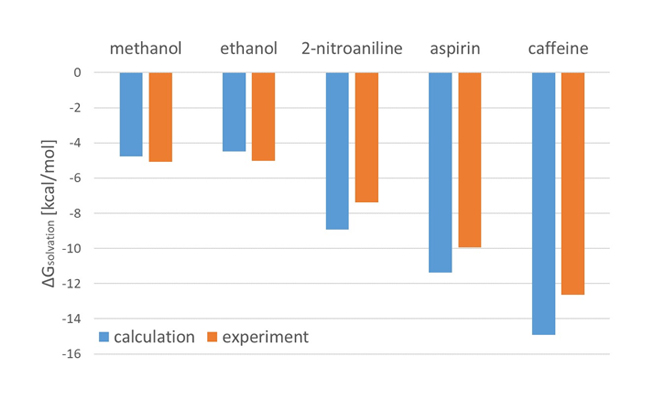 Fig.2:Comparison of calculation results with experimental data for hydration free energy for each solute
Fig.2:Comparison of calculation results with experimental data for hydration free energy for each solute
- Reference
- [1] C. Kobayashi, J. Jung, Y. Matsunaga, T. Mori, T. Ando, K. Tamura, M. Kamiya, and Y. Sugita, J. Compute. Chem. 38, 2193-2206 (2017). https://doi.org/10.1002/jcc.24874
- [2] J. Jung, T. Mori, C. Kobayashi, Y. Matsunaga, T. Yoda, M. Feig, and Y. Sugita, WIREs Comput. Mol. Sci., 5, 310-323 (2015). https://doi.org/10.1002/wcms.1220
- [3] D. L. Mobley, J. P. Guthrie, J. Comput. Aided Mol. Des. 28, 711–720 (2014). https://doi.org/10.1007/s10822-014-9747-x


