- Quantum Chemistry / DFT
- Mechanical / Viscosity / Viscoelasticity
- Interface / Phase Separation / Particle Dispersion
- Materials Science
[Case study] Molecular level adhesion
Objectives and Methods
In industrial materials used in automobiles and aircraft, metal parts are being replaced by plastic in order to reduce weight. However, metal parts are still used in some parts, so the bonding technology between different materials such as metal and plastic is becoming more important.
Adhesion between inorganic surfaces and molecules involves multiple phenomena such as adsorption by intermolecular forces, charge transfer, and chemical bond formation. In such a case, analysis using first-principles calculations is one candidate. Adhesion phenomena have been analyzed from the molecular level using DFT [1]. Here, we introduce an example of a similar calculation using SIESTA.
Figure 1 shows the model used for the calculations. PMMA is modeled as a trimer.The state of PMMA on the gold surface was created by classical MD. NEB calculations were carried out at the KBM/DZP level. The C-NEB (Climb image NEB) option was not used in this calculation because it is assumed that there is no transition state.
Figure 2 shows the calculation results. The energy change before and after adsorption is 2.2 eV. This energy change includes the energy of intermolecular forces and the energy of the structural change of PMMA molecules. From the molecular structure shown in the figure, we can confirm the change in side chain structure during adsorption.
Since there are various possible structures of adsorbed polymers, it is necessary to use multiple initial structures in practical studies. By using SIESTA's NEB calculations, it is possible to compare the differences in inorganic materials, surface structures, and polymers.
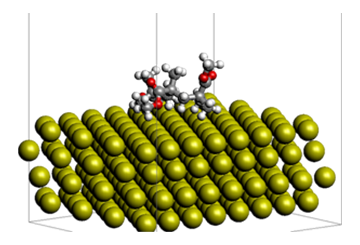 Fig.1 Adsorption state of Au(111)-PMMA
Fig.1 Adsorption state of Au(111)-PMMA
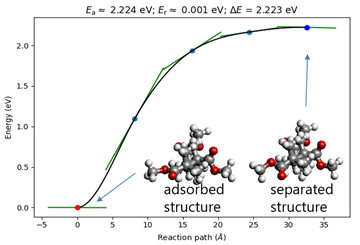 Fig.2 NEB calculation of adsorption process of PMMA on gold surface
Fig.2 NEB calculation of adsorption process of PMMA on gold surface
- Reference
- [1] T. Semoto, Y. Tsuji and K. Yoshizawa, Bull. Chem. Soc. Jpn. Vol. 85, 672678 (2012)


