- Quantum Chemistry / DFT
- Mechanical / Viscosity / Viscoelasticity
- Optical / Electrical / Magnetic
- Materials Science
[Analysis Example] Lithium-ion batteries Graphite anode swelling
Evaluation of Li ion insertion into graphite anode using SIESTA
Carbon materials used for the anode of lithium-ion batteries utilize a compound in which lithium is inserted into layered carbon, called graphite interlayer material. The graphite interlayer material, LiC6, is formed by removing Li ions from LiCoO2, the cathode, and inserting Li ions into the layered carbon through charging. Although this process is reversible, the insertion of Li ions is expected to cause the volume of the electrode to expand or change its mechanical properties.
This phenomenon was analyzed and evaluated using SIESTA, a first-principles calculation engine. Figure 1 shows a graphite-only model image (left) and a LiC6 model image (right) assuming complete desorption of Li ions after relaxation analysis. The results of lattice constants and volume change after relaxation analysis are shown in Table 1.
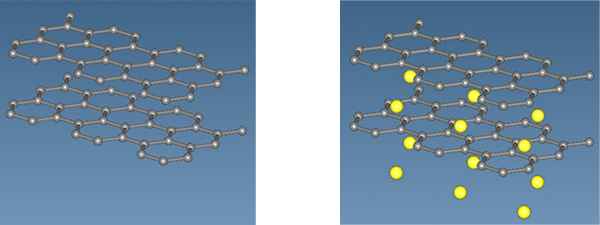 Fig.1 Adsorption state of Au(111)-PMMA
Fig.1 Adsorption state of Au(111)-PMMA
Table 1 shows that LiC6 has 7.5% elongation in the c-axis direction and 10% increase in volume when taken as the interplane distance of graphite as a standard. This means an increase of 2.3% in the ab-plane direction as well as in the c-axis direction.
The elastic stiffness of both models, obtained from the analysis with applied strain, is shown in Table 2; with the exception of C33, LiC6 shows an overall decrease in elastic modulus compared to graphite. For C33, graphite is about 60% of LiC6. The interlayer bonding in graphite is due to van der Waals forces, while in LiC6, weak ionic bonding due to Li insertion is considered to occur in the c-axis direction.
Next, Figure 2 shows the results of comparing the partial density of states of the two materials. Comparing the Fermi level of both materials, it can be seen that the Fermi level is pushed up in LiC6 compared to graphite due to the increase in the number of electrons per unit cell caused by Li ion insertion. The Fermi level of LiC6 is located at a larger density of states than that of graphite, indicating that LiC6 is more metallic than graphite.
Table 1. Comparison of lattice constants and volumes of graphite and LiC6
| Interlayer distance (Å) | Interlayer distance ratio | Volume ratio | |
|---|---|---|---|
| Graphite | 3.48 | 1.00 | 1.00 |
| LiC6 | 3.74 | 1.08 | 1.10 |
Table 2. Elastic stiffness of graphite and LiC6
| Graphite(GPa) | LiC6(GPa) | |
|---|---|---|
| C11 | 1030.8 | 908.6 |
| C22 | 1030.8 | 908.6 |
| C33 | 38.7 | 60.5 |
| C12 | 168.1 | 143.1 |
| C44 | -3.6 | 11.2 |
| C55 | -3.6 | 11.2 |
| C66 | 500.6 | 442.1 |
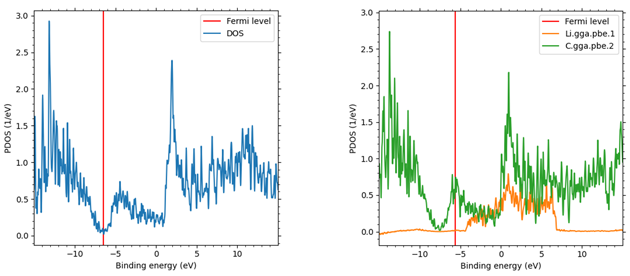 Figure 2. Comparison of density of partial states of graphite (left) and LiC6 (right) after relaxation. Red lines indicate Fermi levels; for LiC6, the contribution of each element is shown.
Figure 2. Comparison of density of partial states of graphite (left) and LiC6 (right) after relaxation. Red lines indicate Fermi levels; for LiC6, the contribution of each element is shown.


