- DPD / Mean Field
- Reptation Dynamics
- Mechanical / Viscosity / Viscoelasticity
- Materials Science
[Analysis Example] Simulation of viscoelasticity using DPD with entanglement effect
DPD Simulation Considering the Entanglement Effect of Polymers
Dissipative Particle Dynamics (DPD) is one of the coarse-grained molecular simulations, which has been widely applied to phase separation phenomena because it employs a soft potential and includes hydrodynamic effects. On the other hand, DPD cannot deal with entanglement motion of polymer chains because particles are crossable each other.
By applying the method described in reference [1] to the VSOP of J-OCTA, we simulated viscoelasticity considering the entanglement of polymer chains. By setting up a virtual bonding potential (Slip-Spring) between polymers, as shown in the figure below, this emulates the entanglement point. After a certain number of steps of the usual DPD time progression, the Slip-Spring is made to move in a sliding motion, and this process is repeated.
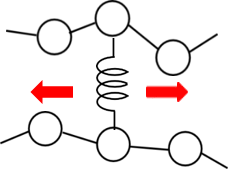 Fig. 1 Slip-Spring set up between polymer chains
Fig. 1 Slip-Spring set up between polymer chains
Figure 2 shows the calculated results, showing the storage modulus G'' and loss modulus G'' of monodisperse polystyrene. The three graphs are for different molecular weights, and the results are all in good agreement with the experimental values.
Although J-OCTA has rheology engines such as NAPLES and PASTA, similar calculations can be performed by using VSOP's DPD (similar functions are currently included in COGNAC).
In addition, DPD is expected to be applied to component interactions and filler dispersion systems as a unique feature of DPD. Cross-linked rubber materials can also be a target. It is recommended to select and use the area in which each engine has strengths.
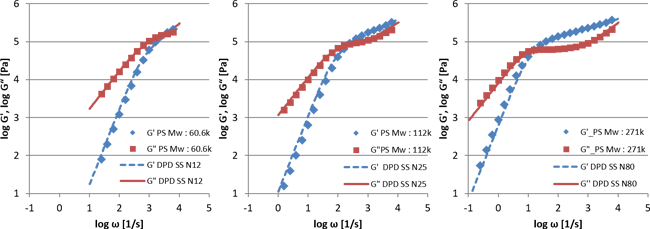 Figure 2: G' and G'' of polystyrene by DPD with entanglement effect.
Figure 2: G' and G'' of polystyrene by DPD with entanglement effect.
Left: molecular weight 60.6k, Middle: molecular weight 112k, Right: molecular weight 271k
- Reference
- [1] Y. Masubuchi, M. Langeloth, M. C. Böhm, T. Inoue, and F. Müller-Plathe, Macromolecules, 2016, 49, 23, 9186-9191


