- Quantum Chemistry / DFT
- Other Properties
- Materials Science
Formation energy of reaction
Purpose and method
The oxidation reaction energies (ΔE) of the three metals shown in Figure 1 were calculated.
Mg + 1/2 O2 → MgO
Si + O2 → SiO2
2Al + 3/2 O2 → Al2O3
The reaction energy (ΔE) is calculated from the following formula and corresponds to the formation energy of oxide. The calculation models for oxidation of Mg are shown in Figure 2. ΔE is obtained from the difference between the energy of the reactants and the energy of the products. The calculation was performed using SIESTA, and PBE was applied to the functional and DZP (EnergyShift = 0.2 eV) was applied to the basis function. (MeshCutoff: 300.0 Ry, kgrid_cutoff: 10.0 angstrom)
E(Mg) + 1/2 E(O2) = E(MgO) + ΔE
E(Si) + E(O2) = E(SiO2) + ΔE
E(2Al) + 3/2 E(O2) = E(Al2O3) + ΔE
 Figure 1. Simulation model of oxide
Figure 1. Simulation model of oxide
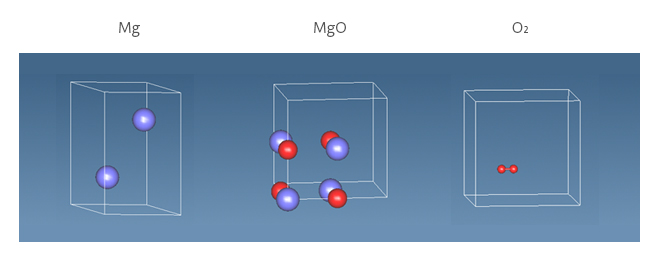 Figure 2. Simulation models for reaction energy of MgO
Figure 2. Simulation models for reaction energy of MgO
Simulation result
The results are shown in Figure 3. All of results were in good agreement with the reference values. This indicates that SIESTA can evaluate the energy change during the reaction.
* The reference value is the enthalpy of oxide formation (ΔH = ΔE + ΔPV) and not exactly the same meaning as the calculated value, but it is compared as an approximate value because the contribution of PV is considered to be small in the case of solids.
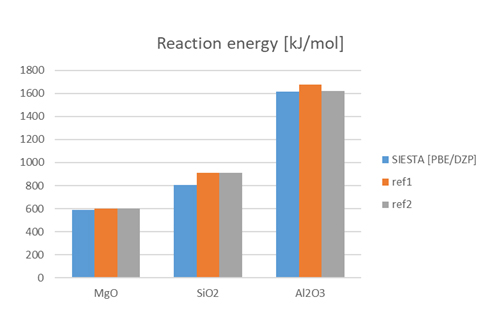 Figure 3. Comparison of calculation results of reaction energy by SIESTA with references
Figure 3. Comparison of calculation results of reaction energy by SIESTA with references
ref1: https://doi.org/10.1155/2014/120840
ref2: NIST Chemistry Webbook (https://doi.org/10.18434/T4D303)


