- Quantum Chemistry / DFT
- Small molecule penetration / diffusion / adsorption
- Materials Science
[Case study] Interaction between zeolite and gas molecules
Objective and methods
LSX zeolite is an X-type zeolite with a Si-Al ratio of 1.0. Li-LSX is known as an oxygen PSA (Pressure Swing Adsorption) adsorbent, that selectively adsorbs N2 in air when the encapsulated ion is Li.
Here, we constructed a zeolite slab model as shown in Fig. 1 in order to analyze the interaction of Li-LSX with N2 and O2 molecules by DFT calculations using SIESTA. The boundary with the vacuum region of the slab was terminated by hydrogen.
The interaction energies between Li ions and gas molecules at the site reported to be the main adsorption site for N2 [1] were then calculated by varying the ion-molecule distance. The interaction energies were evaluated at the KBM/DZP level calculation and were corrected for basis set superposition error (BSSE) using the Counterpoise method. Two directions of N2 molecular axes were considered: vertical (Z) and horizontal (X) as shown in Fig. 1.
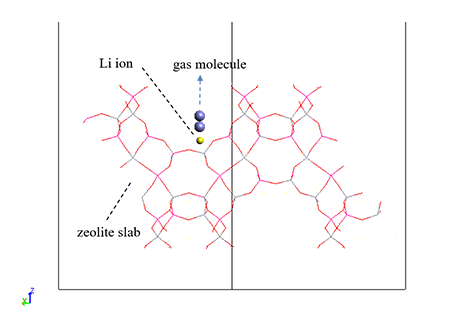 Fig.1 Li-LSX slab model used in this study and the alignment of the gas molecule
Fig.1 Li-LSX slab model used in this study and the alignment of the gas molecule
Fig. 2 shows the interaction energies of N2 and O2 with Li-LSX plotted against the Li-molecule distance. The calculated results show that the interaction energy of the N2 molecule is negatively larger than that of the O2 molecule, indicating that it is strongly adsorbed. It shows that N2 molecules tend to be adsorbed on Li ions in an arrangement where the molecular axis is parallel to the Z direction. This is thought to be due to the charge distribution of N2 molecule, resulting in quadrupole.
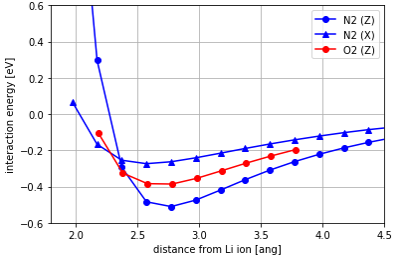 Fig. 2 Interaction energies between Li-LSX and N2/O2 molecules.
Fig. 2 Interaction energies between Li-LSX and N2/O2 molecules.
- Reference
- [1] Yoshida, Hirano, and Nakano, Kagaku Kogaku Ronbunshu, 30, 461 (2004)


