Simulation of lithium-ion batteries
With the prospect that the commercialization of all-solid-state batteries will be delayed until the late 2020s or later, research and development of batteries for EVs is shifting toward exploring a wide range of candidate material possibilities. As a result, there are growing expectations for materials simulations from the perspective of exploring a wide range of materials, including all-solid-state electrolytes.
In this article, we introduce examples of evaluation and investigation by simulation for anode/cathode/electrolyte and manufacturing process, which are the components of lithium-ion batteries.
1.Anode Analysis
It is well known that the practical application of lithium-ion batteries was driven by Dr. Yoshino's discovery of carbon material as a anode material in contrast to LiCoO2, which was known as a cathode material at the time. The carbon material used for the anode is a graphite interlayer compound, in which lithium is doped into layered carbon. In practical use, charging removes Li ions from LiCoO2 and doping Li ions into layered carbon produces LiC6, a graphite interlayer compound (the reverse process occurs during discharge). This process is theoretically a reversible process between the cathode and anode of Li ions during charging and discharging, but the absorption and desorption of Li ions is expected to cause volume expansion and shrinkage and a change in elastic modulus at the electrode.
This situation was confirmed by analysis using SIESTA, a first-principles calculation engine. Figure 1 shows a model image of graphite doped with Li ions as LiC6 after charging before relaxation analysis (left) and a model image of graphite only when Li ions are assumed to have been completely desorbed after discharge (right). Table 1 shows the relaxation analysis for the model in Figure 1, showing the interlayer length, volume change, and c33, the strength of the interlayer bonds. The results show that during Li ion absorption, there is a swelling of about 8% in interlayer length and about 10% in volume. The c33, which indicates the strength of correlative bonds, is reduced by about 40% in graphite compared to LiC6. Figure 2 shows the difference in the electronic states of graphite and LiC6 in terms of their respective density of states (red lines indicate Fermi levels). In Figure 2, the density of states of LiC6 is on the left and the density of states of the graphite layer is on the right. The difference in the Fermi level between the two (change from a gap state to a gapless state) indicates that LiC6 tends to be more metallic than graphite.
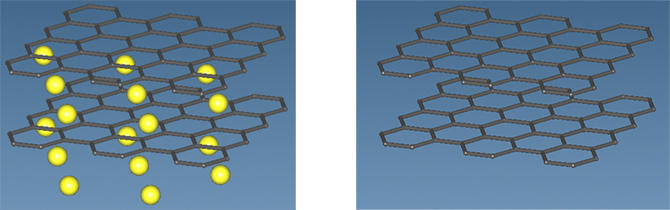 Figure 1. Model of LiC6 (left) and graphite (right) before relaxation calculations
Figure 1. Model of LiC6 (left) and graphite (right) before relaxation calculations
Table 1. Comparison of lattice constants and C33 between graphite and LiC6
| Interlayer distance (Å) | Interlayer distance ratio | Volume ratio | C33(GPa) | |
|---|---|---|---|---|
| Graphite | 3.48 | 1.00 | 1.00 | 38.7 |
| LiC6 | 3.74 | 1.08 | 1.10 | 60.5 |
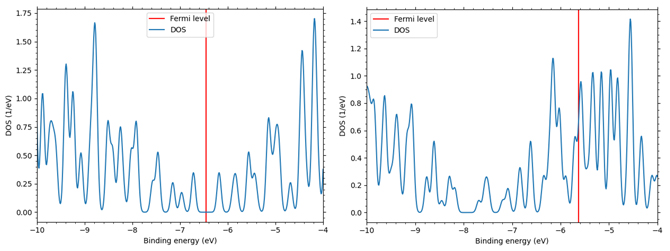 Figure 2. Density of electronic states of graphite (left) LiC6 (right) after relaxation calculation
Figure 2. Density of electronic states of graphite (left) LiC6 (right) after relaxation calculation
There has also been active research and exploration of new anode materials to improve the properties of lithium-ion batteries. Since the capacity of a lithium-ion battery is determined by the capacity of the anode, materials other than carbon-based materials that can absorb a large amount of Li have also been considered. It is known that an anode composed of Si has a theoretical capacity that is 10 times higher than that of conventional carbon-based materials. However, the volume expansion of the Si anode is extremely large (400%) when Li ions are absorbed, and the collapse of the anode due to volume change is a major development issue.
In reference [1], first-principles calculations using SIESTA have reported the relationship between stress and strain occurring in the processes of Li absorption and desorption on an anode composed of amorphous Si, as well as the lithiation (metallization) of Si anode atoms. In this analysis, by controlling the concentration of Li as an external chemical potential, the absorption and desorption processes on amorphous Si are simulated. The analysis shows that the stress history with the Li concentration on the horizontal axis shows hysteresis, indicating that irreversible changes occur in the processes of Li absorption and desorption. In terms of mechanical properties, a decrease in Young's modulus and etc. has been observed due to lithiation of anode atoms caused by Li entering the Si anode [2]. Li absorption leads not only to shape changes seen in swelling, but also to Si-Si bond breaking and Si-Li formation. Electronically, the transition from the semiconducting state to the metallic state of the Si anode has been confirmed through changes in the density of states at the Fermi level [1].
2.Cathode Analysis
In the cathode active material, changes in elastic modulus are also expected to occur due to the absorption and desorption of Li ions. In simulations, for example, mechanical properties such as Young's modulus and bulk modulus of Li1-xCoO2 have been obtained by first-principles calculations[3][4]. According to these results, it has been reported that the volume modulus in CoO2 (x=1) with the Li ions completely desorbed is about half that of LiCoO2 (x=0). In the simulations, a supercell is defined and analyzed by combining several unit cells of Li1-xCoO2 so that the value of 1-x, which indicates the fraction of Li, is the simplest integer ratio.
The bulk modulus was calculated using the EOS (equation of state) function included in the SIESTA Modeler, a first-principles calculation engine (Fig. 3). Physically, Young's modulus and Poisson's ratio or bulk modulus and shear modulus can be obtained by finding the stiffness tensor of the crystal. In simulations, these values can be obtained through analysis of stresses and elastic energies obtained by performing shape optimization calculations (relaxation calculations) after applying strain.
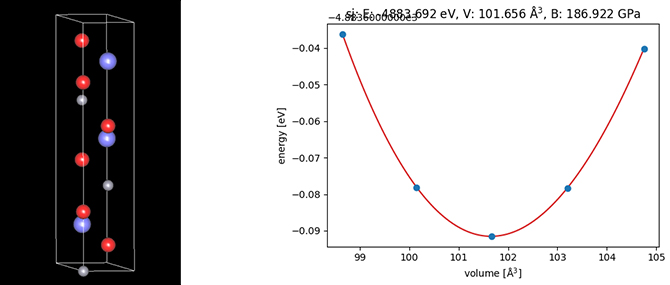 Figure 3. Crystal model of LiCoO2 and elastic modulus analysis using elastic energy
Figure 3. Crystal model of LiCoO2 and elastic modulus analysis using elastic energy
3.Electrolyte Analysis
Electrolytes currently being studied for all-solid-state batteries can be classified into three types: sulfide-based, oxide-based, and polymer-based. Currently, sulfide-based electrolytes have the highest ionic conductivity and are considered to be close to practical application. However, in batteries for EVs, degradation at the electrode interface due to volume change caused by Li ion absorption/desorption has become an issue, and practical application is expected to begin in the late 2020s. For this reason, oxide-based materials are also being considered, although they are not as good as sulfide-based materials in terms of ionic conductivity.
One of the candidates under consideration is LiZr2(PO4)3 (LZP), which belongs to the NASICON type. The ion mobility can be obtained from the diffusion constant through the Nernst-Einstein equation at room temperature. First-principles MD calculations were performed to obtain diffusion constants for LZP, and the results are shown in Figure 4 as an Arrhenius plot. For comparison with the literature [5], the temperature range is set to 673-1773 K. The calculation results show that in the region above 1000 K, there is a quantitative trend consistent with the literature [4]. On the other hand, in the temperature range below 1000 K, the values are about one order of magnitude larger than those in the literature [5]. In detail, an anomalous diffusion effect due to the trapping of Li ions was observed in this temperature region. Since the present calculation takes about 10% of the analysis time compared to the literature [5], we consider that the anomalous diffusion effect has a smaller effect, and as a result, the diffusion constant is pushed up, which is the reason for the discrepancy in the temperature range below 1000 K.
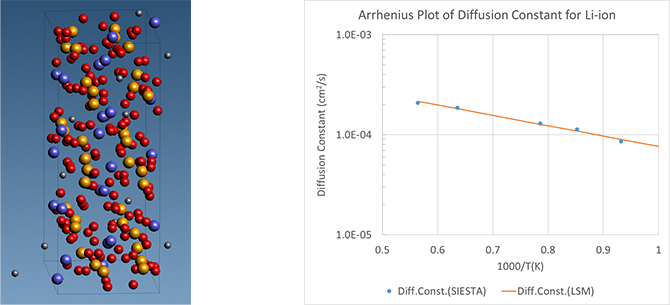 Figure 4. Supercell of LZP during the relaxation process (left) Arrhenius plot of diffusion constant per temperature (right)
Figure 4. Supercell of LZP during the relaxation process (left) Arrhenius plot of diffusion constant per temperature (right)
For the estimation of the diffusion constant by simulation, "MD-GAN" [6], which predicts the results of a long-time simulation by machine learning from the results of a short-time simulation, has been shown to be effective. This will be one of the main analysis methods in the future, especially since it is difficult to predict long-time dynamics with first-principles MD.
In addition, research is being conducted on liquid solvents for dissolving high concentrations of Li salt, which are several times higher than in conventional liquid lithium-ion batteries. In order to dissolve high concentrations of Li salt, a solvent with a high dielectric constant is required to promote the ionization of Li ions. Such solvents include propylene carbonate, sulfolane, and succinonitrile. Their relative permittivity is characterized by the intramolecular electric dipole moment and the intra-atomic electronic polarization.
These contributions are calculated by J-OCTA molecular dynamics simulations and molecular orbital calculations such as Gaussian, and the results of evaluating the relative permittivity by the Onsager/Kirkwood equation are shown in Figure 5.
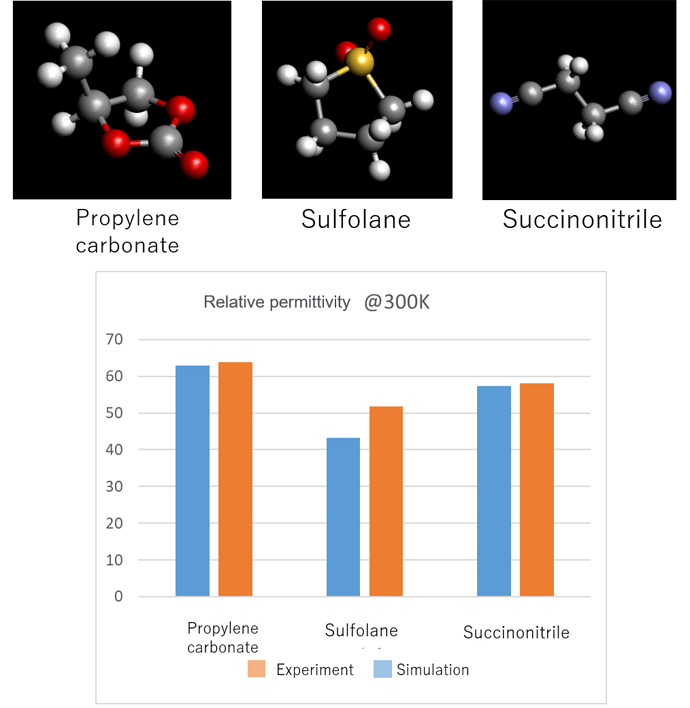 Figure 5. Relative permittivity of PC/C4H8O2S/N2C4H4
Figure 5. Relative permittivity of PC/C4H8O2S/N2C4H4
4.Production Process Analysis
The slurry coating process is one of the core technologies in the manufacturing process of lithium-ion batteries. This is a very important process because the thickness of the cathode and anode films and their thickness ratio during the coating process affect the battery's capacity/rate characteristics, etc. In the coating process, the active material, binder, and organic solvent are mixed into a metal foil that serves as the current collector. In the coating process, a slurry (fluid with suspended solid particles) of active material, binder, and organic solvent is coated on a metal foil that serves as a current collector, and then the solvent molecules are evaporated by a drying process.
In Reference [7], a coarse-grained model of active material, binder, and organic solvent was created using the coarse-grained molecular dynamics method included in J-OCTA to simulate the evaporation process of organic solvent.
Figure 6 shows the time evolution of the coated film due to evaporation of solvent molecules. It can be seen that with the progress of time, the evaporation of solvent molecules proceeds from the region near the surface, and a porous structure is created.
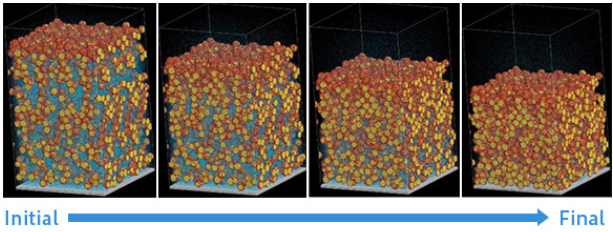 Figure 6. Formation of electrode structure by solvent evaporation
Figure 6. Formation of electrode structure by solvent evaporation
5.Conclusion
Some phenomena that occur in batteries are clearly visible, such as the swelling of the Si anode, but their origin can be traced back to physical changes on the electronic scale, such as the lithiation of the Si anode, which is a multiscale physical phenomenon. J-OCTA provides multi-scope simulation technology for multi-scale phenomena in batteries from various viewpoints. J-OCTA provides multi-scope simulation technology for multi-scale phenomena occurring in batteries from various perspectives.
- Reference
- [1] Kejie Zhao, Georgios A. Tritsaris, Matt Pharr, Wei L. Wang, Onyekwelu Okeke, Zhigang Suo, Joost J. Vlassak, and Efthimios Kaxiras, Nano Lett. 2012, 12, 4397−4403
- [2] Kejie Zhao, Wei L. Wang, John Gregoire, Matt Pharr, Zhigang Suo, Joost J. Vlassak, and Efthimios Kaxiras, Nano Lett. 2011, 11, 7, 2962–2967
- [3] Yue Qi, Louis G. Hector, Jr., Christine James, and Kwang Jin Kim, Journal of The Electrochemical Society, 161 (11) F3010-F3018 (2014)
- [4] Linmin Wu and Jing Zhang, JOURNAL OF APPLIED PHYSICS 118, 225101 (2015)
- [5] Yusuke Noda, Koki Nakano, Hayami Takeda, Masashi Kotobuki, Li Lu, and Masanobu Nakayama, Chem. Mater. 2017, 29, 8983−8991
- [6] K. Endo, K. Tomobe and K. Yasuoka. Proc. Conf. AAAI Artif. Intell., 2018.
- [7] https://www.j-octa.com/cases/caseA36/


